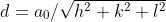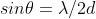釋意
crystalline material:
A uniform substance made up of a three dimensional, grid-like repeating pattern.
(materials have long-range order arrangement of its atoms)
single crystal:
A crystalline material that is composed of only one crystal.
polycrystalline material:
A crystalline material which is composed of many smaller crystals or grains, randomly oriented.
Amorphous :
A material that displays only short range order.
assume的意思
Why do some materials assume(呈現(xiàn)水慨,這里不是假設的意思) an amorphous structure?
The kinetics of the manufacturing process do not allow a regular or crystalline pattern to form.
crystal structure, lattice, basis, unit cell
A lattice is a collection of points arranged in a spatial pattern so each point has a relation its surrounding points that is valid for all other points as well.
A unit cell is the smallest part of a lattice that shows the repeating pattern of the lattice.
The basis is composed of atoms with a certain relationship spatial relationship.
The crystal structure is the combination of the lattice and basis and shows how the atoms of a crystal are arranged with respect to each other throughout the entire thing.
# of lattice points per cell:
(如果1atom/lattice, 就是#atoms/cell)
SC:1 BCC:2 FCC:4 HCP:2
coordination number:
SC:6 BCC:8 FCC:12 HCP:12
atomic radius與lattice parameters的關系:
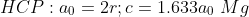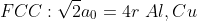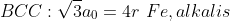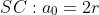
\rho, PF
 = \frac {m}{v} = \frac {atoms/cell \cdot (M/NA)}{V_{unit cell}}) = \frac{atoms/cell \cdot V_{atom}}{V_{unit cell}})
7種晶格
cubic, hexagonal, tetragonal, orthorhombic, monoclinic, rhombohedral, triclinic
面和方向坐標的區(qū)別
[方向]<方向族> [100][-100]相反 [100][200]相同
(平面){平面族} (100)(-100)相同 (100)(200)不同
面:
planar destiny: 個數(shù)/面積
packing fraction: 面積/面積
線:
repeat distance: 1/LD
linear density (LD): 個數(shù)/長度
packing fraction: LD*直徑
Diffraction tech for crystal structure analysis